What is surface finishing? What does it do?
Surface treatment refers to a variety of processes and techniques applied to the surface of a material or object to modify its properties, appearance, or behavior. The goal of surface treatment is to enhance the performance, durability, functionality, and aesthetics of the material or object in question.
There are numerous reasons for applying surface treatments, and they can be broadly categorized into the following purposes:
1. Functional Improvement: Surface treatments can alter the properties of a material to make it more suitable for specific applications. For example, surface treatments can make materials more resistant to corrosion, wear, abrasion, or chemical reactions. They can also enhance properties such as hardness, conductivity, or thermal resistance.
2. Aesthetic Enhancement: Surface treatments can improve the appearance of an object by providing a desired color, texture, or finish. Processes like painting, plating, and coating can give objects a polished or decorative look.
3. Adhesion and Bonding: Surface treatments are often used to improve the adhesion properties of materials. Properly treated surfaces can facilitate better bonding between materials, such as in adhesive bonding or welding processes.
4. Cleaning and Preparing: Before some manufacturing processes, surfaces need to be cleaned, degreased, or prepared to ensure proper adhesion of coatings or other materials.
5. Biocompatibility and Medical Applications: In medical and biological contexts, surface treatments are used to make materials compatible with biological systems. For example, medical implants can be treated to reduce the risk of rejection by the body.
6. Reducing Friction: Surface treatments like lubrication or adding low-friction coatings can reduce the friction between surfaces, making them more efficient and durable.
7. Optical Enhancement: Surface treatments can alter the reflective, refractive, or diffusive properties of materials for optical purposes. This is often seen in lenses, mirrors, and other optical components.
8. Heat Treatment: A type of surface treatment that involves controlled heating and cooling of materials to alter their mechanical properties, such as hardness and toughness.
9. Protection: Coatings and treatments can protect surfaces from environmental factors such as UV radiation, moisture, chemicals, and more, thus extending the lifespan of the object.
Examples of surface treatment methods include:
1. Painting and Coating: Applying layers of paint, varnish, or protective coatings to enhance appearance and protection.
2. Plating and Electroplating: Depositing a layer of metal onto a surface to enhance corrosion resistance, conductivity, or aesthetics.
3. Anodizing: Creating an oxide layer on metals (like aluminum) to improve corrosion resistance and color stability.
4. Heat Treatment: Altering the microstructure of a material through controlled heating and cooling to improve its mechanical properties.
5. Surface Etching: Chemically or mechanically altering the surface texture for aesthetic or functional purposes.
6. Laser Surface Treatment: Using lasers to modify surface properties like hardness, texture, and wear resistance.
In this article we will discuss about electroplating.
1. What is electroplating?
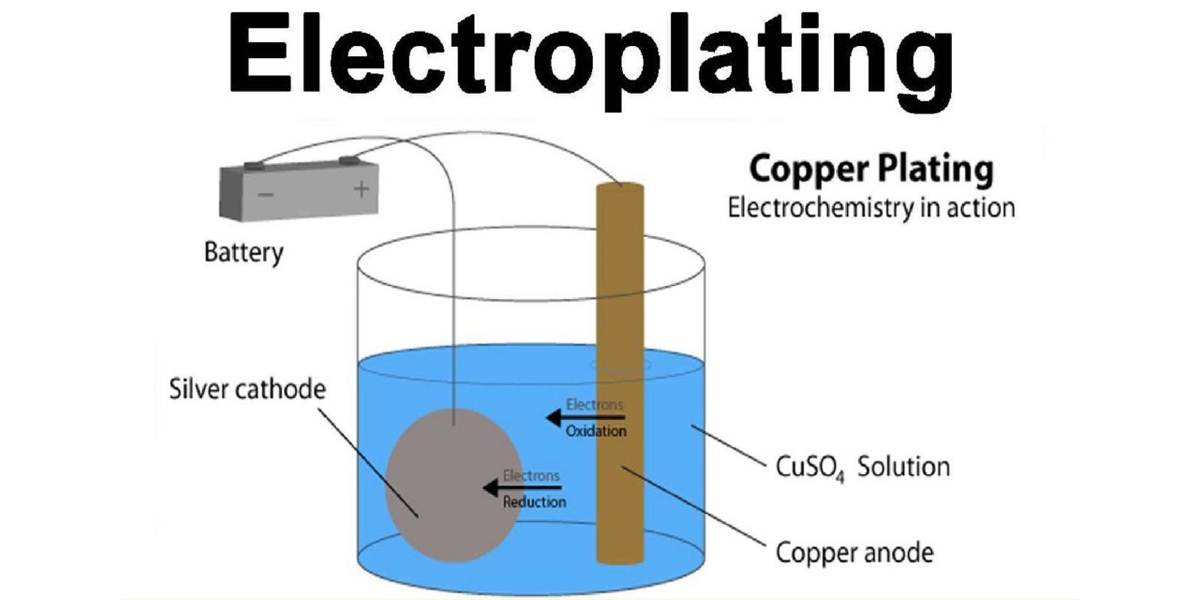
Electroplating is a surface treatment process in which a thin layer of metal is deposited onto a conductive object's surface through the use of an electric current. The object to be plated is immersed in a solution containing metal ions of the plating material, and an electric current is passed through the solution. This causes the metal ions to migrate and adhere to the surface of the object, forming a uniform and adherent metal coating.
The thickness and quality of the plated layer can be controlled by adjusting factors such as the current density, plating time, and the composition of the electrolyte solution. Electroplating is commonly used to improve the appearance, corrosion resistance, and conductivity of objects. It's widely employed in industries such as jewelry making, automotive manufacturing, electronics production, and decorative coating applications.
Different types of metals can be used for electroplating, including gold, silver, nickel, chromium, zinc, and more. Each metal provides specific properties to the plated object, such as enhanced aesthetics, improved durability, or electrical conductivity.
2. Formation process of plating
The process of electroplating involves the deposition of a thin layer of metal onto a conductive object's surface using an electric current.
l Preparation of the Object:
The object to be plated, known as the cathode, must be thoroughly cleaned to ensure proper adhesion of the plated layer. Any dirt, grease, or contaminants on the surface can interfere with the plating process.
l Selection of Electrolyte Solution:
The choice of metal for plating determines the type of electrolyte solution to be used. The electrolyte solution contains metal ions of the plating material. For instance, if you want to gold-plate an object, the solution will contain gold salts.
l Preparation of Electrolyte Solution:
The electrolyte solution is prepared by dissolving the appropriate metal salts in water or another solvent. This creates a solution with positively charged metal ions.
l Setup of Electroplating Bath:
The electrolyte solution is placed in a tank or container, often referred to as the electroplating bath. The object to be plated is submerged in the bath and connected to the negative terminal of the power source (cathode).
l Anode Setup:
An anode, typically made of the same metal as the plating material, is also placed in the bath and connected to the positive terminal of the power source. The anode gradually dissolves as metal ions are needed to maintain the concentration in the solution.
l Application of Electric Current:
When the electric current is applied, metal ions in the electrolyte solution are attracted to the cathode (object to be plated). They gain electrons and get reduced, forming a solid metal layer on the cathode's surface. This layer gradually builds up over time.
l Metal Deposition and Plating:
As the current flows, metal ions are continuously reduced at the cathode's surface, forming a uniform layer of plated metal. The thickness and quality of the plated layer depend on factors such as current density, plating time, and solution concentration.
l Control and Monitoring:
Throughout the plating process, parameters like current intensity, temperature, and plating time are closely monitored and controlled to ensure the desired quality and thickness of the plated layer. These factors impact the final appearance and characteristics of the plated object.
l Completion and Finishing:
Once the desired plating thickness is achieved, the object is removed from the bath. Depending on the intended use, additional finishing steps like polishing, buffing, or coating may be performed to enhance the appearance and properties of the plated surface.
3. What are the roles and benefits of plating?
Roles of Plating:
Enhanced Aesthetics: Plating can improve the appearance of an object by providing it with a lustrous, reflective, or colorful surface. This is particularly important in industries like jewelry, decorative art, and luxury goods.
Corrosion Protection: Plating can form a protective barrier on the surface of an object, preventing direct contact with corrosive agents such as moisture and chemicals. This helps extend the lifespan of the object and maintains its appearance.
Wear and Abrasion Resistance: Plated layers can improve the resistance of objects to wear, abrasion, and friction. This is valuable in applications where objects experience regular contact or movement, such as machine parts and tools.
Electrical Conductivity: Plating can enhance the electrical conductivity of a surface, making it useful in applications where electrical contact or conductivity is required, such as electronic components and connectors.
Solderability: Plated surfaces often have improved solderability, making them easier to solder in electronic assembly processes.
Hardness Enhancement: Some plated materials can significantly increase the surface hardness of objects, improving their resistance to scratches and dents.
Chemical and Environmental Resistance: Plated layers can provide resistance to chemical reactions and environmental factors, making them suitable for use in challenging environments.
Medical and Biocompatibility Applications: Certain plating materials are biocompatible, making them suitable for medical implants and devices that interact with the human body.
Benefits of Plating:
Cost-Effective: Electroplating is generally a cost-effective method for achieving desired surface properties compared to other alternatives like solid metal fabrication.
Customizability: Plating processes can be tailored to achieve specific characteristics such as thickness, composition, and finish, allowing for customization to suit particular needs.
Uniform Coating: Electroplating results in a uniform and even coating on complex shapes and surfaces, ensuring consistent performance and appearance.
Preservation of Base Material: Plating allows you to impart desirable properties to the surface while preserving the core material's attributes. For example, you can have a strong base material with a corrosion-resistant plated layer.
Design Flexibility: Plating allows for creative design possibilities by combining different materials and finishes to achieve unique looks and performance characteristics.
Improved Durability: Plating can significantly enhance the durability of objects, extending their usable life and reducing maintenance requirements.
4. What industries and parts are plating used in?
Exterior Trim: Chrome plating is used to enhance the appearance and corrosion resistance of various exterior parts such as bumpers, grilles, and emblems.
Wheel Rims: Plating is applied to wheel rims for both decorative and corrosion-resistant purposes.
Engine Components: Plating can improve wear resistance and corrosion protection on engine parts like pistons, cylinder heads, and valve components.
Electronics Industry:
Connectors: Plating enhances the electrical conductivity and corrosion resistance of connectors used in electronic devices and circuit boards.
Printed Circuit Boards (PCBs): Plating processes are used to create conductive traces and protect the PCB from oxidation.
Jewelry and Accessories:

Jewelry: Precious metals like gold, silver, and platinum are often used for decorative and corrosion-resistant plating on jewelry items.
Watches: Plating can enhance the appearance and durability of watch cases, bands, and clasps.
Aircraft Components: Plating is used to improve corrosion resistance and other properties of parts in the aerospace industry, including fasteners, landing gear components, and engine parts.
Implants: Biocompatible plating materials are used for medical implants like orthopedic devices and dental implants.
Surgical Instruments: Plating can provide corrosion resistance and easy sterilization for surgical instruments.
Consumer Goods:

Household Appliances: Plating is used on components of appliances like faucets, handles, and decorative elements to enhance appearance and durability.
Cutlery: Plating can provide corrosion resistance and improved aesthetics for utensils and cutlery.
Fashion and Design:
Clothing Accessories: Plating is applied to buttons, buckles, and fashion accessories for decorative purposes.
Decorative Art: Plating is used in sculptures, art pieces, and decorative items to achieve desired aesthetics.
Military and Defense:
Firearms: Plating can provide corrosion resistance and improved appearance to firearm components.
Military Hardware: Plating is used on various military equipment to enhance durability and performance.
Telecommunications:
Antennas and Towers: Plating can be used on communication equipment to improve conductivity and protection against environmental factors.
Solar Panels: Plating is used to create conductive layers on solar panels to enhance their efficiency.
Marine Industry:
Boat Hardware: Plating can protect boat hardware from the corrosive effects of saltwater.
Machine Parts: Plating can improve the wear resistance and longevity of various machine components.
5. What materials can plating work on?
Electroplating can work on a wide range of materials, provided that the material is conductive and can be properly prepared for the plating process.
Metals:
Steel
Stainless Steel
Copper
Brass
Bronze
Aluminum
Nickel
Silver
Gold
Platinum
Palladium
Rhodium
Zinc
Alloys:
Various alloys of the metals mentioned above can also be plated, such as bronze alloys, brass alloys, and more.
Base Materials:
Non-metallic objects that have been coated with a conductive layer can also be plated. For example, plastic objects with a conductive coating can be electroplated.
It's important to note that while many materials can be plated, the success of the plating process depends on factors such as the material's composition, surface condition, and its compatibility with the plating solution. Some materials might require special pretreatment steps to ensure proper adhesion of the plated layer.
Certain materials are more commonly plated due to their properties and applications. For example:
Precious Metals: Gold, silver, and other precious metals are often plated for their aesthetic appeal and corrosion resistance in jewelry, electronics, and decorative items.
Base Metals: Base metals like copper and nickel are frequently used as underlayers for plating processes, providing a foundation for other layers and improving adhesion.
Non-Ferrous Metals: Non-ferrous metals such as aluminum and its alloys can be plated for enhanced corrosion resistance and other desired properties.
Iron and Steel: Plating is used on iron and steel to improve corrosion resistance, appearance, and wear resistance in applications ranging from automotive parts to household items.
Non-Conductive Materials: While not directly plated due to their lack of conductivity, non-conductive materials like plastics and ceramics can be coated with a conductive layer (usually through processes like vacuum metallization) before undergoing plating.